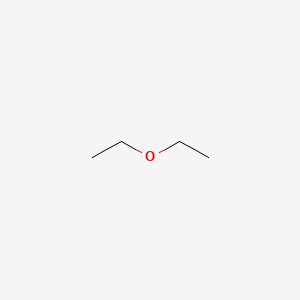
Diethyl Ether Bond Line Structure

The composition and structure of ether.
Diethyl ether bond line structure. The carbon atoms in the chemical structure of diethyl ether are implied to be located at the corner s and hydrogen atoms attached to carbon atoms are not indicated each carbon atom is considered to be associated with enough hydrogen atoms to provide the carbon atom with four bonds. A carrier for a hair care product containing polyvinylpyrrolidone was prepared with 77 04 g 70 aq dimethyl ether 14 11 g dimethyl ether 1 09 g co2. Closely related to alcohol both through history and chemistry is ether ethyl ether diethyl ether a compound obtained from alcohol by the action of oil of vitriol sulfuric acid. The oxygen bonded with two carbon atoms and containing two unpaired electrons forms a bent shape.
Diethyl ether is formed by four tetrahedron ax4 and one bent shape ax2e2. Ethyl ether also known as diethyl ether well known anesthetic generally referred to as simply ether an organic compound belonging to a large group of compounds called ether. The preparation of alcohol spirit of wine vinic alcohol ethanol ethyl alcohol by fermentation dates to antiquity. Ether c2h5 2o or c4h10o cid 3283 structure chemical names physical and chemical properties classification patents literature biological activities.
Diethyl ether or simply ether is an organic compound in the ether class with the formula c 2 h 5 2 o sometimes abbreviated as et 2 o see pseudoelement symbols it is a colorless highly volatile sweet smelling ethereal odour flammable liquid it is commonly used as a solvent in laboratories and as a starting fluid for some engines. It was formerly used as a general anesthetic until. An aerosol mixt contains dimethyl ether 28 97 38 5. Its molecular structure consists of two ethyl groups connected by an oxygen atom as in c2h5oc2h5.
The bond angles in a bent shape are 104 5 degrees. What happens if you inhale diethyl ether. There are two tetrahedron on each side of the oxygen molecule. Structure properties spectra suppliers and links for.