Diethyl Ether Nmr Peaks

2o as a solvent the accepted reference peak δ 0 is the methyl signal ofthe sodium salt of3 trimeth ylsilyl propanesulfonicacid one crystal ofthis was added toeach nmr tube.
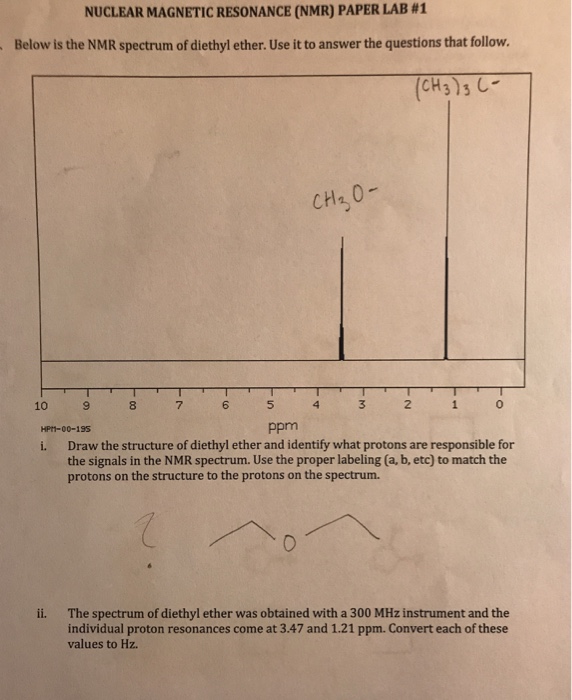
Diethyl ether nmr peaks. The two ethyl groups in diethyl ether show as a single absorbance since the molecule has a plane of symmetry that is both ethyl groups are chemically and magnetically equivalent. Tags diethyl ether 60 29 7 1 h nmr related products ethylparaben 120 47 8 ir1 ethylparaben 120 47 8 ir2 ethylparaben 120 47 8 1 hnmr ethylparaben 120 47 8 13 cnmr ethylparaben 120 47 8 ir3 ethylparaben 120 47 8 ms ethylparaben 120 47 8 raman 2 2 dichlorodiethyl ether 111 44 4 13 cnmr 2 2 dichlorodiethyl ether 111 44 4 raman 2 2 dichlorodiethyl ether 111 44 4 ms 2 2 dichlorodiethyl. Diethyl ether ch 3 t 7 1 21 1 11 1 09 1 11 1 12 1 18 1 17 ch 2 q 7 3 48 3 41 3 38 3 26 3 42 3 49 3 56 diglyme ch. Nmr chemical shifts of common.
Dynamic proton 1h and carbon 13c nmr chemical shift tables with various solvents. 1997 62 7512 7515 doi. Diethyl ether or simply ether is an organic compound in the ether class with the formula c 2 h 5 2 o sometimes abbreviated as et 2 o see pseudoelement symbols it is a colorless highly volatile sweet smelling ethereal odour flammable liquid it is commonly used as a solvent in laboratories and as a starting fluid for some engines. Extra peaks in a variety of commonly used nmr solvents in the hope that this will be of assistance to the practicing chemist.
This material has several disadvan tages however. Which of the following combinations of peaks appears in the 1h nmr spectrum of 2 3 dimethylbutane. It is not volatile so it cannot be readily eliminated ifthe sample has tobe recovered. Occasionally inorder to distinguish between peaks whose assignment was ambiguous afurther1 2ílofaspecificsubstratewere addedandthespectrarunagain.
Nmr chemical shifts of common laboratory solvents as trace impurities gottlieb h. Which of the following combinations of peaks appears in the 1h nmr spectrum of diethyl ether ch3ch2och2ch3. A triplet and a quartet. If we use a common laboratory solvent diethyl ether acetone dichloromethane ethanol water etc to dissolve our nmr sample however we run into a problem there many more solvent protons in solution than there are sample protons so the signals from the sample protons will be overwhelmed.